What is water made of?
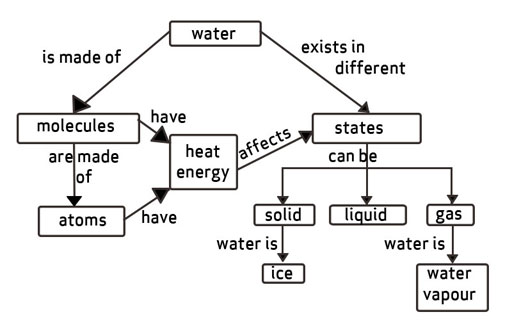
Atoms are the smallest building blocks of everything in the Universe, and you've undoubtedly heard of them. We are all formed up of atoms that are bound together (or "bonded," as physicists call it). Molecules are made up of atoms that are bound together.
Atoms make up everything. The smallest molecule of an element, such as oxygen or hydrogen, is called an atom. Molecules are formed when atoms come together. Two hydrogens (H) atoms and one oxygen (O) atom make up a water molecule. That's why H2O is occasionally used to refer to water. There are billions of water molecules in a single drop of water.
Physical properties of water
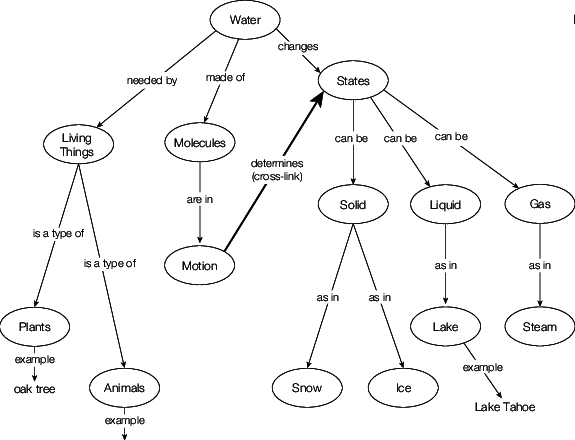
Water has several essential physical characteristics. Although these features are well-known due to water's ubiquitous existence, the majority of water's physical properties are rather unusual. Water has an extraordinarily high viscosity, heat of vaporization, and entropy of vaporization, and surface tension which may be attributed to the significant hydrogen bonding connections found in liquid water, given the low molar mass of its constituent molecules. Solid water is much less dense compared to liquid water due to the open structure of ice, which allows for maximal hydrogen bonding. This is a relatively rare scenario amongst common substances.
Chemical properties of water
A molecule of water has the chemical formula that is H2O, which consists of two hydrogens (H2) atoms bonded to one oxygen atom (O). Atom electrons particles that have a negative charge forms a bond with one another. Hydrogen is less effective in keeping them close to it than oxygen. The water molecule is negatively charged towards the oxygen atom and positively charged towards the hydrogen atom. Water molecules prefer to stick together like magnets because opposites attract.
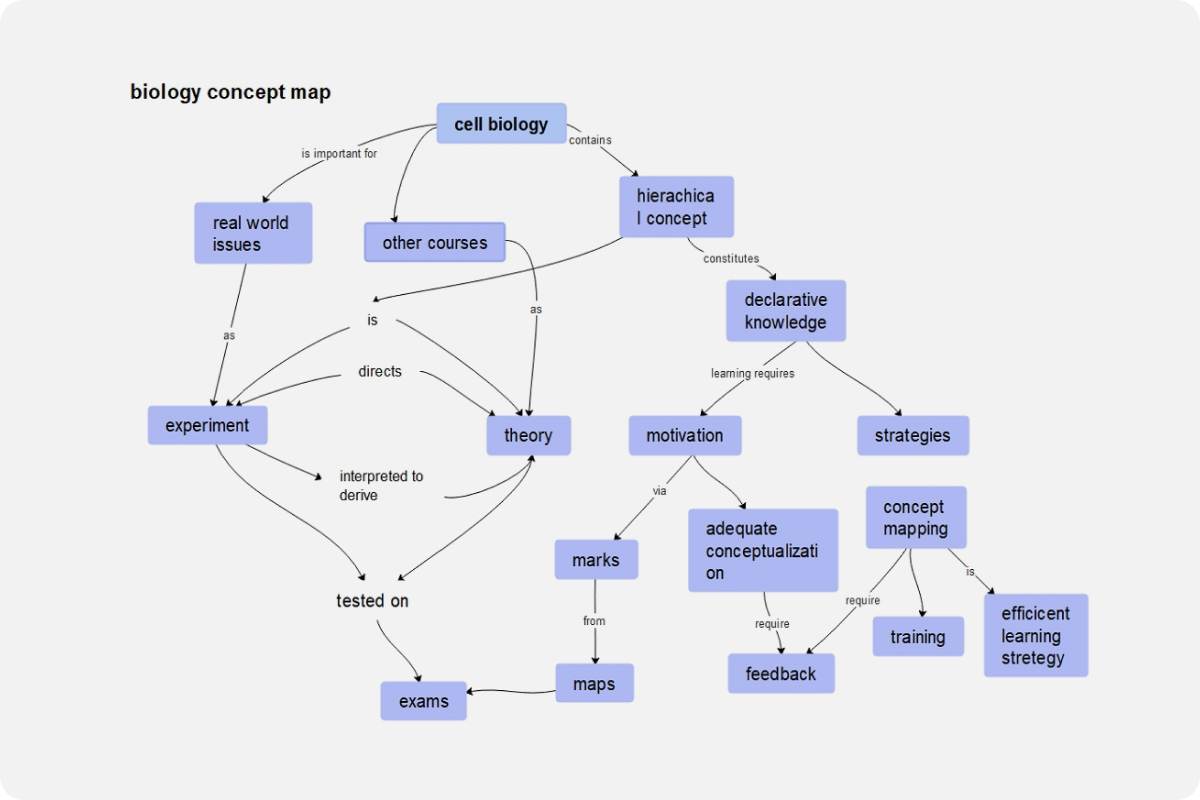
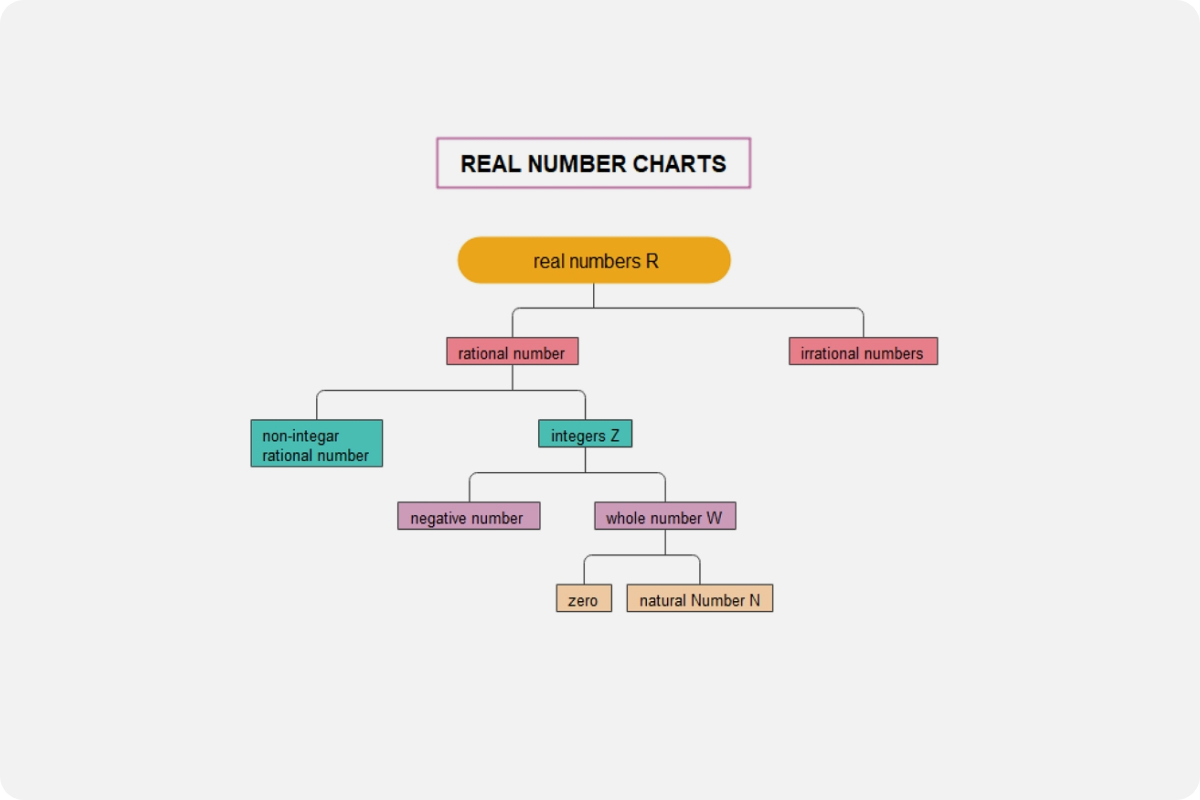
Water concept map examples
Example 1
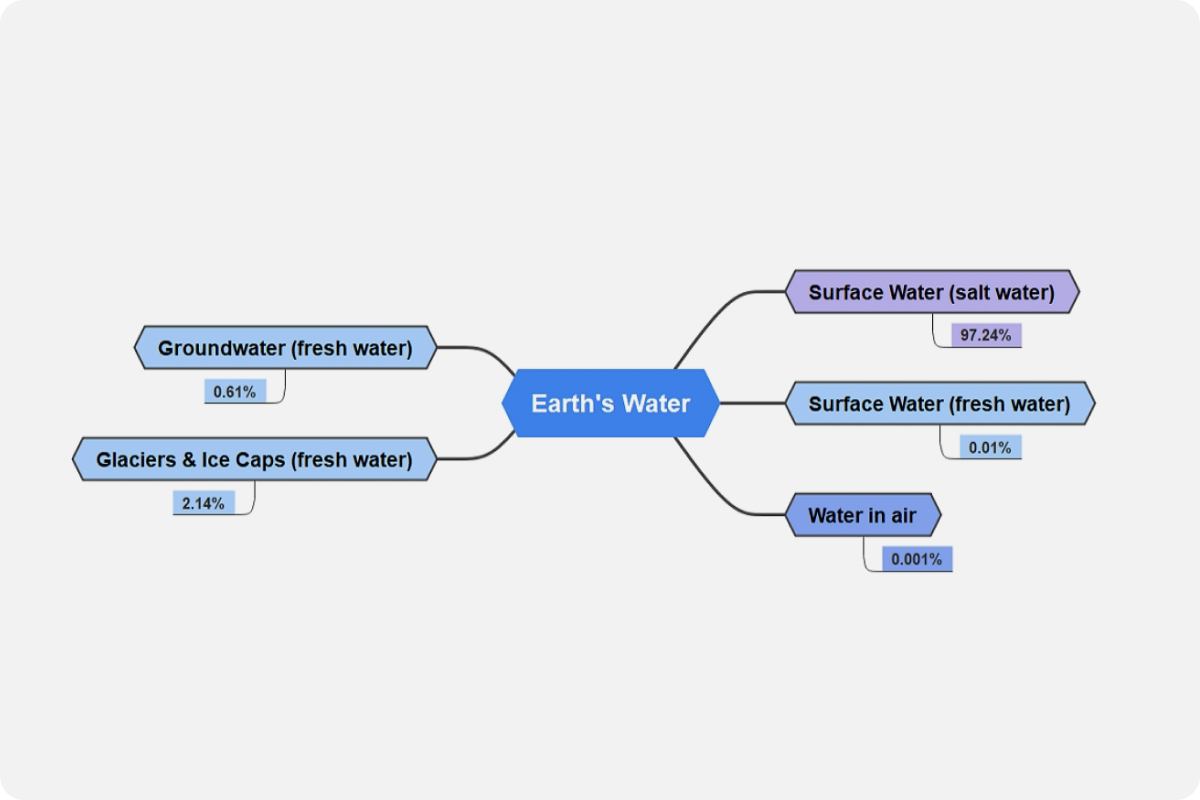
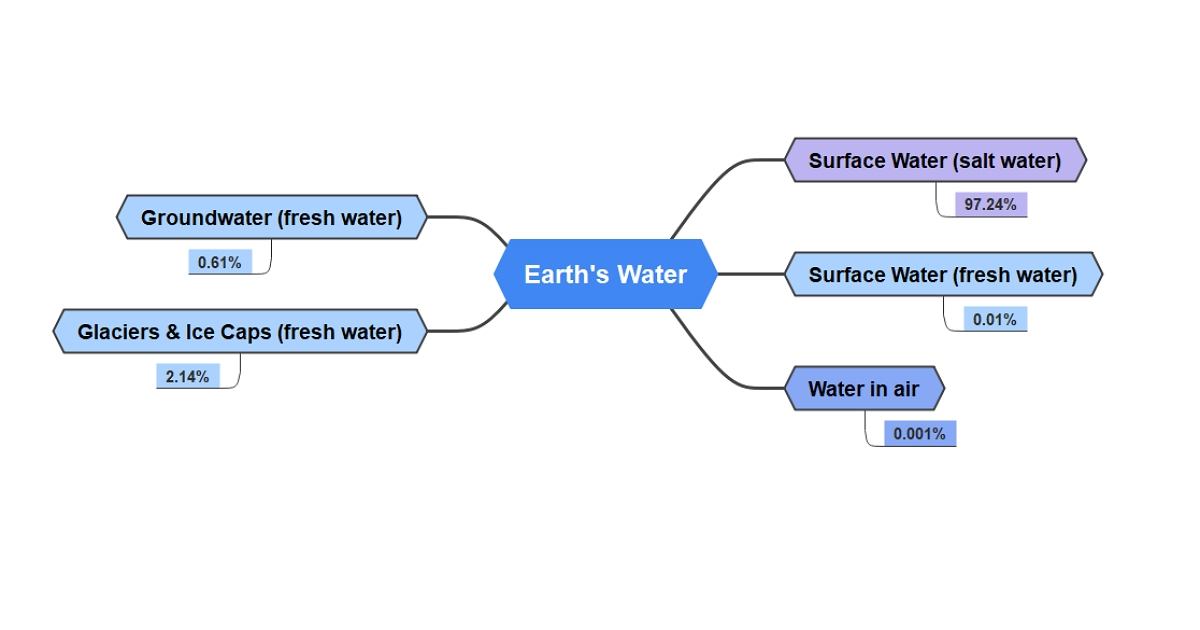
Water, the primary source of life on Earth, cycles continually through the water cycle, one of the planet's most powerful processes. Between the ocean, the atmosphere, and the land, water flows inexorably. The amount of water in, on, and above our world is finite, which means that it does not actually reduce or even increase. Saltwater makes up around 97 percent of the water on our globe, whereas freshwater makes up less than 2.14%. The majority of the world's fresh water is frozen in glaciers, ice caps, and aquifers. Freshwater that is freely accessible to humans to satisfy our requirements makes up less than 1% of the Earth's water, and the majority of that water is supplied by precipitation.